PATIENT INFORMATION: Cardiovascular and Respiratory System Implants
What … for? (Indications for implants)
Implants for the heart help with
- Structural Heart Diseases as
1.1 Partial Heart Substitutes
1.2 Hybrid Heart Assistive Devices
1.3 Total Artificial Heart
1.4 Vessel (substitute, support) - Abnormal heart rhythms (artificial pacemaker)
Different distinction criteria:
– Pulsatile heart supporting Systems
– Non-pulsatile heart supporting Systems
1. Structural Heart Diseases
1.1 Partial Heart Substitutes (VHD=Valvular Heart Disease)
- Severe aortic stenosis (AS)
- Aortic Dissection (AD)
- Bicuspid Aortic Valve
- Rheumatic MS (mitral Stenosis)
- MR (mitral regurgitation)
- TR (Tricuspid Valve Disease)
- Pulmonic Valve Disease
- Mixed Valve Disease
- Patients with prosthetic valves
- Symptomatic heart disease due to either severe native calcific aortic stenosis or failure
- Dilated ascending aorta in combination with valve pathology
- Left ventricular dysfunction
- Aortic valve disease causing hemodynamic burden sufficient to affect left ventricular structure or function
- Enlargement of left ventricle (parachute implant)
Patients who cannot tolerate surgery for aortic valve replacement may be candidates for a less invasive approach called TAVI or TAVR (Transcatheter Aortic Valve Replacement).
Summary of recommendations for prosthetic valve choice (Nishimura)
A bioprosthesis is recommended in patients of any age for whom anticoagulant therapy is contraindicated, cannot be managed appropriately, or is not desired.
A mechanical prosthesis is reasonable for AVR or MVR in patients <60 y of age who do not have a contraindication to anticoagulation.
A bioprosthesis is reasonable in patients >70 y of age. Either a bioprosthetic or mechanical valve is reasonable in patients between 60 y and 70 y of age.
1.2 Hybrid Heart Assistive Devices
- To bridge the time to heart transplantation or to permanently replace the heart in case heart
- Transplantation is not possible
- Left Ventricular Assist Device (LVAD)
- Right Ventricular Assist Device (RVAD)
- Biventricular Assist Device (BiVAD)
1.3 Total Artificial Heart
- To bridge the time to heart transplantation or to permanently replace the heart in case heart transplantation is not possible
- Total artificial heart (TAH), Prototypes, Single Case Experiences
1.4 Vessels
- Intracranial aneurysms
- Reduced vessel (e.g. coronary, carotid) diameter
- Closed vessels
- AS (Aortic Stenosis)
- AR (Aortic Regurgitation)
Replacement of the aortic valve by a pulmonary autograft (the Ross procedure), may be considered in young patients when VKA anticoagulation is contraindicated or undesirable (Nishimura).
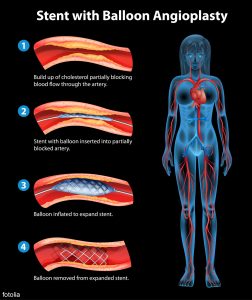 stent angioplasty procedure
stent angioplasty procedure
2. Abnormal heart rhythms (artificial pacemaker)
- Bradycardia, arrhythmia, heart block, congenital heart diseases.
- If heartbeat is too slow or irregular.
- May be combined with an internal cardioverter defibrillator (ICD)
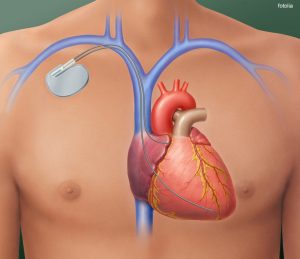 heart with implanted pacemaker
heart with implanted pacemaker
Generally a multidisciplinary approach by a specialized team is recommendable.
Numbers
USA: American Heart Association (USA/AHA):
– 480.000 PCI procedures (2014, AHA)
– 3.244 Heart transplants (2014, AHA)
– 106.000 Valve replacements (2006)
In the USA in 2019, coronary events are expected to occur in about 1,055,000 individuals, including 720,000 new and 335,000 recurrent coronary events.
Pacemakers worldwide (2009), 1.14 Mio (2014, statista), USA 351.000 (2014, AHA)
LVADs worldwide about 4,000 implanted (total until 2000)
1 of every 2.9 deaths in the United States is related to heart diseases (AHA, 2011)
From 2006 to 2016, the US death rate from CVD decreased by 18.6% and from coronary heart disease by 31.8%.
GERMANY (DGTHG): 2019: 100,446 surgeries (without pacemaker, more)
Times
Structural therapies: very different acc. to type and scope of the surgery.
Pacemaker: hospital stay several hours until days.
Contraindications
- behavioral
- missing, low compliance
- medical
- Comorbidities precluding the expected benefit
- Frailty Mortality Scores . eg Euroscore (STS risk estimate)
- Procedure specific impediments
- Heart infections
- Low life expectancy
- during surgery n/a
Risks
- during surgery
- Impracticability
- problems with general anesthesia
- death
- short term no benefit
- long term no benefit
- restrictions
- accompanying medical treatment, control regiment.
- artificial pacemaker: avoid intense magnetic fields, contact sports
- electric interference may occur (e.g. at magnetic resonance imaging MRI, radiofrequencies, transcutaneous electrical nerve stimulation TENS, therapeutic radiation, diathermy, electric tools)
more
Failures n/a, many varieties
Material
- artificial heart
- structural implants (valves, widener, opener…..)
- autologous
- pulmonal valve (e.g. “Ross” operation)
- allogenic
- human valves
- xenogenous
- bovine pericardium
- Sorin pericardial valves
- Tissue heart valves
- Collagen
- bovine pericardium
- alloplastic
- Stents after coronary, peripheral angioplasty (balloon angioplasty) leaving a stent
- Stainless steel, gold, cobalt-chromium, tantalum
- Nitinol (55%Ni, 45%Ti)
- Nitinol-composite with platinum core
- Silicone
- Shape-memory polymers
- biodegradable
- Polyorthoester
- Polyanhydrids
- Polyester (biodegradable)
- Stents after coronary, peripheral angioplasty (balloon angioplasty) leaving a stent
- autologous
- implantable pacing systemes (pacemaker, defibrillator, cardioverter, CRT Cardiac resynchronization therapy, CRT-D, implantable cardioverter defibrillator (ICD)
- alloplastic
- generator (resistors, diodes, capacitors, semiconductors)
- wired / wireless
- titanium
- battery
- lithium, iodine
- electrodes
- generator (resistors, diodes, capacitors, semiconductors)
- alloplastic
Statistics
Aortic valve operations: 80% of patients had a predicted risk of mortality (PROM) of <4% and actual mortality rate of 1.4%, the remaining 20% had a higher risk (2002 – 2010).
Literature
- basic
- problems
- science/future
Medical Societies see here
Use the information button or the doctor-finder in the medical societies section. If available you´ll find information in your language and according to the region you live.
![]()
![]()
Criticism
Heart stents still overused (NYT, 2013)
Selected Patient Information
Angioplasty Patient Center
Artificial Pacemaker (1) – arrhythmia
Artificial Pacemaker (2) – what is a pacemaker
Artificial Pacemaker (3) – electromagnetic interference
Atrial Fibrillation
Carotid Artery Stent (Medline)
Cholesterol (AHA)
Heart Failure Matters (multilingual)
Heart Valve Disease (University of Michigan)
Implantation eines ICD- oder CRT-Systems (german)
Risks of having a stent (NIH)
Sudden cardiac death (AHA)
Register for Patients
International pacemaker patient identification cards carry information such as patient data (among others, symptom primary, ECG, aetiology), pacemaker center (doctor, hospital), IPG (rate, mode, date of implantation, manufacturer, type) and lead type.
In the register of the registries you can check which ones are available.
If your implant is not covered:
The Implant-Register offers registration of implants online and you can download a printable version for your personal use.
Disclaimer: The information and links and whatsoever shown on this page are compiled with care. However, Implant-Register can´t take any responsibility for the information given, nor their content, nor their up-to-date nature, particularly in interlinked pages. You may help us with your contribution, granting us the decision to publish or not. Be careful with conclusions for yourself, in doubt double-check and consider medical solutions are individual and have to be found with an educated medical person.