Easy Fast S, MF and MTF
 |
|||||||||
| DEUTSCH | |||||||||
IMPLANT REGISTRATION |
 |
sales point | Reg. No.: 06/15/00431/0001 |
||||||
| Easy Fast S, MF and MTF | 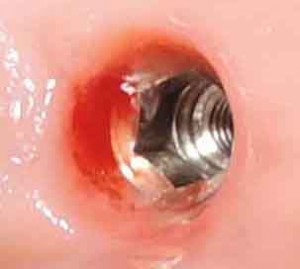 |
 |
 |
||||||
| implant | topview | X-ray | surface | ||||||
| Ο | indication | Implant, compensation of dental root. All standard indications like single tooth replacement, bridges, denture supporting techniques according to the given anatomic preconditions. Easy Fast S MTF recommended for periodontal compromised patients. All cylindrical implants are more recommendable for bone quality 1-3. | |||||||
| Ο | special indication | All standard indications for dental implants, recommended insertion: minimally supra bone level | |||||||
| Ο | contraindication | No contraindications specific to the implant. Cylindrical implants are not recommended for bone quality 4 and in anatomic regions with apical narrowing alveolar process. Regular contraindications of oral implants regarding especially oral hygiene, bone support, prosthetic possibilities. | |||||||
| Ο | implant description | Despite the different external dimensions, our own-brand EasyLine products have identical connecting units. Platform switching is possible. With the aid of these structures, we are able to offer a broad portfolio accompanied by a refined range of prosthetic elements. | |||||||
| Ο | material | Titanium grade 5, Ti6Al4V, material proof acc. EN2900 | |||||||
| Ο | biocompatibility | Basic biocompatibility of titanium, f.i. Donath, K., Kirsch, A., Osborn, J.-F.: Zelluläre Dynamik um enossale Titanimplantate, ZZI, 155, 1984 | |||||||
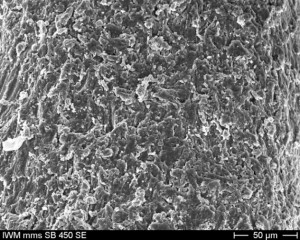
| Ο | implant surface | bone: “Easy” surface, surface magnification, double processing with Al2O3-particles soft tissue: alternatively rough (easy fast S), polished at small platform Switch (easy S MTF), miniscrew Surface (easy fast S MF) |
|||||||
| Ο | sizes, measures | length: 8.5, 10.0, 11.5, 13.0, 16.0mm, diameter: 3.5, 4.3, 5.0mm | |||||||
| Ο | approvals | X | CE-Marking | X | EN ISO 13485 | ||||
| X | EC Directive 93/42/EEC | ||||||||
| Ο | technical parts | connection with abutments: internal hexagon connection abutments: impression post (open and closed tray), lab analog, straight and angulated (15°, 25°), individual posts, titanium and ceramic, locator, special adaptable bar-system. healing caps: 3-7mm, cylindrical and tapered. customized parts: individual adjustable bar for removable suprastructures, individual abutments possible with 3D-planning. |
|||||||
| Ο | manufacturing | own production, subtractive | |||||||
| Ο | key literature | Osseointegrationsverhalten | |||||||
| Ο | directions | company | implant | ||||||
| info@general-implants.com | |||||||||
| regional: represented in Austria, Germany, Iran, Russia, Spain, Turkey. | |||||||||
| Ο | notes of the company | CMF craniomaxillofacial instruments and tools like screws and plates available. Individual production of implants and tools in titanium and ceramic possible, own production. | |||||||
| Ο | last update | 2018-08-14 | |||||||
| registered / this page is approved by the provider | |||||||||
| Implant Registration shows a fully registered implant. This is a summary of the most important parameters of an implant according to our specifications. All implants are recorded according to the same rules in order to create transparency. Further information can be obtained from the manufacturer or the respective national institutions. Information given here are checked thoroughly by “Implant-Register”. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | |||||||||
| No comments yet. | |||||||||

