PATIENT INFORMATION: Hip Endoprosthesis
WHAT … FOR? (INDICATIONS)
Replacement of partial or total hip joint, Osteoarthritis (pain), fractures and more special disease
The criteria for the insertion of joint implants are:
- severe joint pain for months, several days a week or permanently,
- no relief from other treatments such as painkillers and exercise therapies,
- severe impairment of quality of life
- changes in the joint typical of osteoarthritis that are clearly shown by an X-ray examination.
Other reasons may include: limitations in walking and climbing stairs, a misalignment of the legs, an unstable joint or weak muscles, problems walking, kneeling or sitting down, limitations at work, at home and during leisure time, or dependence on support from others.
NUMBERS
370.000 hip endoprostheses, U.S.A. (2014)
239.204 hip endoprostheses, Germany (2018)
309 per 100.000 population (Germany, 2017, statista)
75% total , 12.5% partial hip replacements, 12.5% revisions (Fortune Business Insights)
TECHNIQUE
The type of fixation of the hip endoprosthesis can be different. It can be cementless, cemented or hybrid (i.e. a combination of these).
 hip replacement implant
hip replacement implant
The surgical approach can vary from minimally invasive to larger approaches to the surgical site. This largely depends on the size of the damage and the required surgical effort.
For smaller fractures, so-called “internal fixation” may be sufficient. In this case, the bone fragments are stabilized with screws and maybe pins. The natural joint and the femur are saved.
The partial hip arthroplasty procedure is used when the end of the bone is damaged or displaced. If the acetabulum (socket) is still functional, only the femoral head can be replaced if necessary. A special type is the duo head prosthesis. In this case, an artificial second cup is placed in the still existing natural cup. These procedures can also be useful if a patient’s ability to bear stress is limited for other reasons (e.g. other illnesses, advanced age).
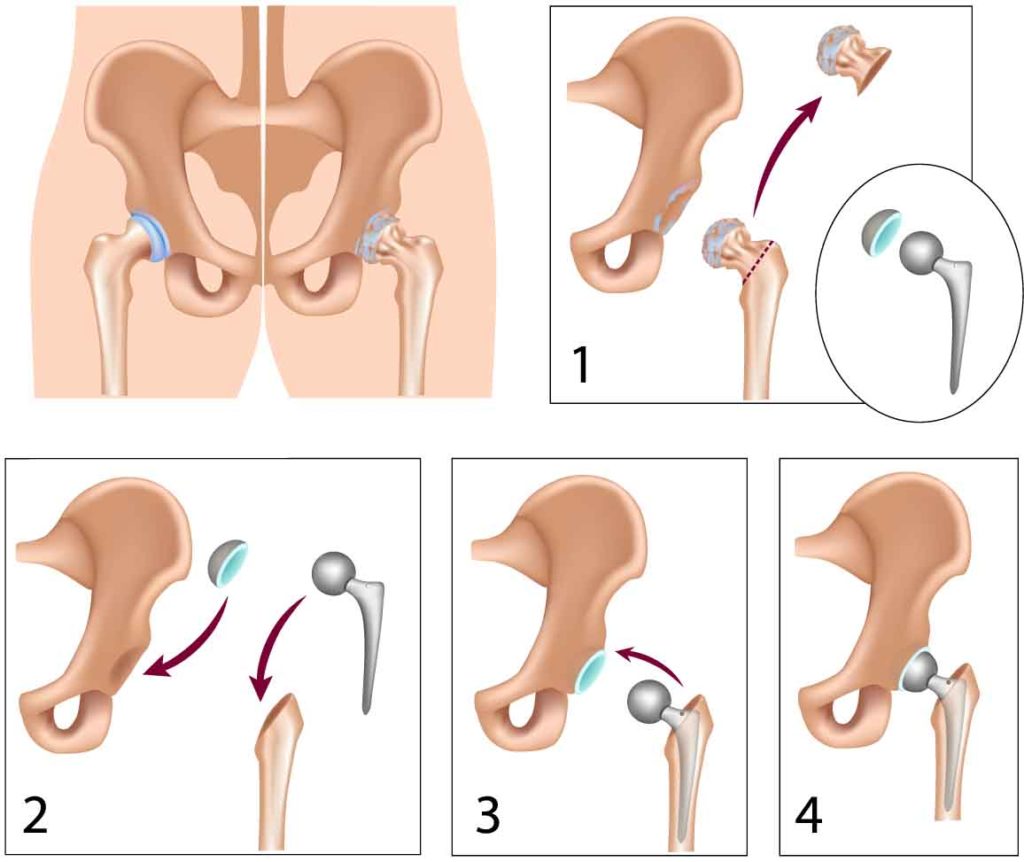
A total hip replacement involves replacing the upper femur and its stem, as well as both the condyle and the socket.
TIMES
Dependend on technique mobilization after 1 -7 days. Rehabilitation after 6-12 weeks dependend on many individual factors.
CONTRAINDICATIONS
Diseases that increase the risk of surgery.
RISKS
short term: Inflammation, thrombosis, hematoma, injury of nerves or vessels, hemorrhagia, metal hypersensitivity, metal toxicity, leg length inequality.
long term: Loosening, dislocation, osteolysis, inflammation, chronic pain, death (<1%).
FAILURES
5% after 1st surgery
MATERIALS AVAILABLE
Titanium (TiAl6V4 a.o.), CoCrMo-alloys, Polyethylen (Teflon), PMMA
STATISTICS
95% more than 10 years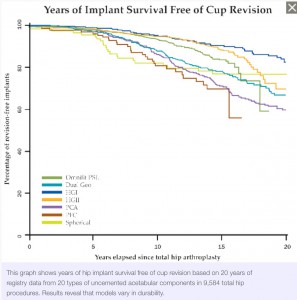
SELECTED PATIENT INFORMATION
Questions to ask your doctor before getting a hip endoprothesis (deutsch)
Endoprothesenregister Deutschland (Patienteninformationen)
LITERATURE
– Basic
Patient Information (manufacturer/Zimmer) (english)
Metal-on-Metal Hip Implants (english, FDA)
Endoprothetik – Zwischen Anspruch und Realität (german)
– Problems
Hip-Replacement-Recalls (Drugwatch)
Selbsthilfegruppe für schadhafte Hüftprothesen (german)
Der Spiegel: Rückruf von Hüftgelenksprothesen (german)
– Science/Future
3D-printed spine
REGISTER (links)
Canadian Joint Replacement Registry (Canada)
Czech National Register of Joint Replacement (NRKN, Czech Republic)Deutschland Endoprothesenregister (german)
Romanian Arthroplasty Register (Romania)
Slovak Arthroplasty Register (Slovakia)
Swedish Hip Arthroplasty Register (swedish)
UK National Joint Registry (UK)
Gelenkersatz: Anwendungsstandards und Register verbessern Qualität (german)
Register und Netzwerke: Zusammenarbeit ist wichtig für den Erfolg (german)
MORE INFORMATION
If your implant is not covered:
The Implant-Register offers registration of implants online (fee) and you can download a printable version for your personal use.
Disclaimer
The information and links and whatsoever shown on this page are compiled with care. However, Implant-Register can´t take any responsibility for the information given, nor their content, nor their up-to-date nature, particularly in interlinked pages. You may help us with your contribution, granting us the decision to publish or not. Be careful with conclusions for yourself, in doubt double-check and consider medical solutions are individual and have to be found with an educated medical person.
