PATIENT INFORMATION: Urogenital System Implants
What … for? (Indications)
Urinary tract implants help at
- female/male stress urinary incontinence (SUI), treatment by bladder string surgery)
- urinary retention
- overactive bladder in patients who have failed or could not tolerate more conservative treatments (Sacral Neuromodulation) and
- Postprostatectomy incontinence (PPI)
- Injection of an implantable material to reduce flow at incontinence
Sex organ implants (only male) help at
- Erectile dysfunction
- Micropenis and
- Repair after removal of testicels
Penile Implants (types)
-
- Non inflatable penile implant
- Two-Piece inflatable penile implant
- Three-piece (multicomponent) inflatable penile implant
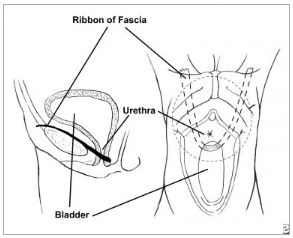
principle of string surgery
Numbers
No reliable statistic numbers.
Penile prosthesis: 20000-30000 devices annualy worldwide (Urology Team, P.A.)
Times n/a
Contraindications
- behavioral
Quite a number of incontinence as well as penis problems don´t need surgical intervention and may be treated on a psychological basis Considering the following facts:
· One in three women who have had a baby experience loss of bladder control.
· One in five children wet the bed at some time.
· One in 100 adults never achieve bladder control at night.
· One in 20 adults experience bladder and bowel control problems - medical
- Incontinence: Severe incontinence is not likely solved with sling procedures, bladder disorders, inadequate tissue integrity at the bladder neck or urethra, active urinary tract infection, pure urge incontinence, prior radiation therapy, cancer.
- Bladder Neurostimulation is not indicated at mechanical obstruction, prostatic hypertrophy, cancer, urethral stricture, no adequate response to test stimulation, unability to operate the tool.
- Penis implants: They are not indicated at psychogenic reasons, diseases of the lower urinary tract, internal erosion or adhesion, low reliability for follow-up care.
- during surgery n/a
Risks
- during surgery
- nerv cutting
- short term
- infection, hemorrhage, urethral obstruction, stress urinary incontinence, urge incontinence, urinary retention
- long term
- sling rejection, perforation
- chronic infection, tearing of skin, scarring, permanent loss of sexual function, loss of sensation in the penis, impotence
- malfunction of implant, penis implants may stay for 4-8 years
- restrictions
- sling surgery: 2-3 days in hospital, 2-4 weeks recovery period, obstipation possible
- penis implant: pain for about 4 weeks. oral medication, driving is prohibited, no sexual activity for 6 weeks.
Failures n/a, too many varieties
Material
- autologous
- fascia lata
- fresh frozen/freeze dried
- solvent-dehydrated
- irradiated
- fat
- fascia lata
- allogenic
- xenogenous
- porcine dermis
- porcine small intestinal mucosa
- alloplastic
- silicone
- polymethylsiloxane
- polyethylene (Marlex®)
- polypropylen (Prolene®)
- acellular collagen matrix (Pelvicol®)
- polyglactin 900 (Vicryl mesh)
- expanded polytetrafluoroethylene PFTEe (Gore-Tex)
- polyethylene terephthalate (Mersilene)
- hydroxyapatite (Coaptite®)
- belladerm matrix graft (penis widening)
- titanium beads with magnetic cores (effectiveness has not been demonstrated)
- implantable bladder pacing system, bladder stimulator
Statistics
- Incontinence surgery: 80-85% success rate (Comiter CV)
- Bladder stimulation/Sacral Nerve Stimulation (SNS)/Percutaneous Tibial Nerve Stimulation (PTNS): 63-64% success rate (Anthem)
- Penis implant: n/a
Literature
- basic
- problems n/a
- science/future n/a
Medical societies see here
Criticism
- Urinary incontinence: n/a
- Penis Enlargement: Does it work?
Selected Patient Information
- Urinary Incontinence (NIH)
- Urinary incontinence – Surgery and procedures (NHS)
- Opinion on the safety of surgical meshes used in urogynecological surgery (EU SCENIHR)
- Penis Enlargement: Myths and Facts (webmd.com)
- Penis enlargement
(Statement: We didn´t find any independend, scientific research data about penis enlargement. Beside surgical interventions there are different options)
Register for Patients
- no specific registry known
- If your implant is not covered:
The Implant-Register offers registration of implants online (fee) and you can download a printable version for your personal use. - in the register of the registries you can check which ones are maybe available concerning your region or topic
Disclaimer
The information and links and whatsoever shown on this page are compiled with care. However, Implant-Register can´t take any responsibility for the information given, nor their content, nor their up-to-date nature, particularly in interlinked pages. You may help us with your contribution, granting us the decision to publish or not. Be careful with conclusions for yourself, in doubt double-check and consider medical solutions are individual and have to be found with an educated medical person.