Easy Torque
 |
|||||||||
IMPLANT REGISTRATION |
 |
discontinued implant | Reg. No.: 06/15/00431/0005 |
||||||
| Ο | indication | Implant, compensation of dental root. Single tooth replacement. | |||||||
| Ο | special indication | Esthetic zone. Especially single tooth replacement. Special requirements regarding ceramic implants. Easy fixing through twisted nail | |||||||
| Ο | contraindication | No contraindications specific to the implant. Regular contraindications of oral implants regarding especially oral hygiene, bone support, prosthetic possibilities. Contraindications on behalf of ceramic implants. | |||||||
| Ο | implant description | Dental two-piece cylindrical ceramic implant with internal connection. | |||||||
| Ο | illustration | 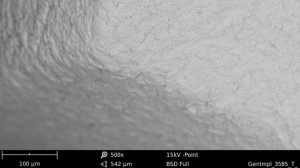 |
|||||||
| implant | surface | ||||||||
| Ο | material | Zirkoniumdioxide, ZrO2 | |||||||
| Ο | biocompatibility | n/a | |||||||
| Ο | implant surface | untreated ceramic surface | |||||||
| Ο | sizes, measures | diameter/length: 3.8: 10, 11.5, 13; 4.3: 8, 10, 11.5, 13; 5.0: 8, 10, 11.5, 13. | |||||||
| Ο | approvals | X | CE-Marking | X | EN ISO 13485 | ||||
| X | EC Directive 93/42/EEC | ||||||||
| Ο | technical parts | connection with abutments: abutment for customizing and gluing. abutments: impression post (open and closed tray), lab analog. |
|||||||
| Ο | manufacturing | own production | |||||||
| Ο | key literature | n/a | |||||||
| Ο | directions | company | implant | ||||||
| info@general-implants.com | |||||||||
| regional: represented in Austria, Germany, Iran, Russia, Spain, Turkey. | |||||||||
| Ο | notes of the company |
|
|||||||
| Ο | last update | 2016-10-09 | |||||||
| registered / this page is approved by the provider | |||||||||
| Implant Registration shows a fully registered implant. This is a summary of the most important parameters of an implant according to our specifications. All implants are recorded according to the same rules in order to create transparency. Further information can be obtained from the manufacturer or the respective national institutions. Information given here are checked thoroughly by “Implant-Register”. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | |||||||||
| No comments yet. | |||||||||

