Easy Cut
 |
||||||||
 |
sales point |
REGISTERED |
Reg. No.: 06/15/00431/0003 |
|||||
| IMPLANT PROFILE | ||||||||
Easy Cut |
 |
|||||||
| implant | surface | |||||||
| Ο | indication | Implant, compensation of dental root. All standard indications for dental rootform implants, especially in small alveolar ridges. | |||||||
| Ο | special indication | All standard indications for dental implants, recommended insertion: defined bone level insertion, defined mucosal attachment zone. Suitable for minimal invasive techniques – for anatomic or physical health compromised patients. Immediate loading for denture bearing structures especially in the mandible possible. Guided surgery available. | |||||||
| Ο | contraindication | No contraindications specific to the implant. In big volume alveolar ridges and in spacious spongious bone conditions as well as in certain esthetic requirements bigger diameter implants should be preferred. Regular contraindications of oral implants regarding especially oral hygiene, bone support, prosthetic needs. | |||||||
| Ο | implant description | Tapered, small diameter, two-piece implant with external connection. | |||||||
| Ο | material | Titanium grade 5, Ti6Al4V, material proof acc. EN2900 | |||||||
| Ο | biocompatibility | Basic biocompatibility of titanium, f.i. Donath, K., Kirsch, A., Osborn, J.-F.: Zelluläre Dynamik um enossale Titanimplantate, ZZI, 155, 1984 | |||||||
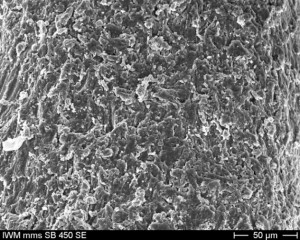
| Ο | implant surface | bone: Easy surface, surface magnification, double processing with Al2O3-particles soft tissue: Surface magnified, mucosal attachment zone |
|||||||
| Ο | sizes, measures | length: length: 14.0/9.5, 16.0/11.5, 18.0/13.5, 20.0/15.5, diameter: 3.4mm | |||||||
| Ο | approvals | X | CE-Marking | X | EN ISO 13485 | ||||
| X | EC Directive 93/42/EEC | ||||||||
| Ο | technical parts | connection with abutments: external hexagon connection. abutments: Straight, adjustable posts, titanium and ceramic, ball retainer, locator, bar-system, custom-made abutments possible. healing caps: Cylindrical hulls (Titanium, 2.5-6.5mm length). customized parts: Individual adjustable bar for removable suprastructures, individual abutments possible with 3D-planning. |
|||||||
| Ο | manufacturing | own production, subtractive | |||||||
| Ο | key literature | Osseointegrationsverhalten | |||||||
| Ο | directions | company | implant | ||||||
| info@general-implants.com | |||||||||
| regional: represented in Austria, Germany, Iran, Russia, Spain, Turkey. | |||||||||
| Ο | notes of the company |
|
|||||||
| Ο | last update | 2018-08-14 | |||||||
| registered / this page is approved by the provider | |||||||||
| Implant Registration shows a fully registered implant. This is a summary of the most important parameters of an implant according to our specifications. All implants are recorded according to the same rules in order to create transparency. Further information can be obtained from the manufacturer or the respective national institutions. Information given here are checked thoroughly by “Implant-Register”. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | |||||||||
| No comments yet. You are welcome to send comments or experiences. HERE, if you received an implant. and HERE, if you placed an implant. |
|||||||||

