Ankylos®
IMPLANT PROFILE |
||||||||
 |
unregistered register here |
|||||||
| n/a | 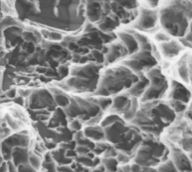 |
||||||
| the implant | topview | X-ray | surface |
| 01 | indication | dental implant, compensation of dental root | ||||||||
| 02 | specific | tissue-care-connection, Acuris cement-free connection | ||||||||
| 03 | contraindication(s) | ◊◊◊◊◊ | ||||||||
| 04 | implant description | ◊◊◊◊◊ | ||||||||
| 05 | specific attributes | ◊◊◊◊◊ | ||||||||
| 06 | material(s) | titanium | ||||||||
| 07 | implant surface | n/a | <picture> | |||||||
| 08 | key literature | ◊◊◊◊◊ | ||||||||
| 09 | notes about the company | n/a | ||||||||
| 10 | approvals | CE-Marking | FDA-Approval | clean implant | |||||
| learn more about approvals, certifications, authorities | ||||||||
| No comments yet. You are welcome to send comments or experiences. HERE, if you received this implant. and HERE, if you placed this implant. |
||||||||
| Received this implant? You can register it HERE (fee). Placed this implant? You can register it HERE (fee). |
||||||||
| current as of 2023-01-25 | ||||||||
| unregistered / this page is not approved by the provider / unregistered implants have no professionnal lines or advertising | ||||||||
| This “Implant Profile” shows a non-registered implant. This is a most comprehensive description of the important parameters of an implant. Information given here are checked thoroughly by “Implant-Register” and is not approved by the manufacturer/the company. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Implants may not be available in all countries. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | ||||||||
IMPLANT PROFILE |
 |
unregistered register here |
||||||
| 01 | application | dental implant, compensation of dental root | ||||||
| 02 | specific | tissue-care-connection, Acuris cement-free connection | ||||||
| 03 | company | denstply sirona Inc. | ||||||
| 04 | illustration | |
|
 |
||||
| implant | connection | surface | ||||||
| 05 | material | titanium | ||||||
| 06 | year of introduction | 1987 | ||||||
| 07 | approvals | X | CE-Label | X | FDA-approval | |||
| n/a | clean implant | |||||||
| 08 | last update | 2018-07-13 | ||||||
|
this page is not approved by the provider |
||||||||
| Information given here are checked thoroughly by “Implant-Register”. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. | ||||||||
