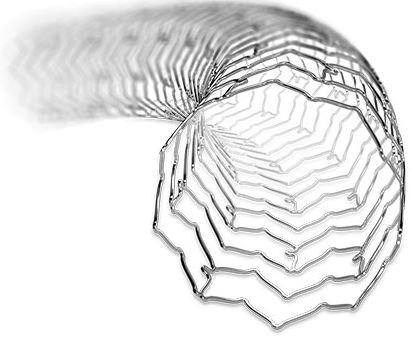
XIENCE Sierra™ Stent
IMPLANT PROFILE |
||||||||
 |
unregistered register here |
|||||||
 |
|||||||
| <the implant> |
| 01 | indication | implant, improving coronary artery luminal diameter, including those high risk for bleeding and diabetes mellitus, de novo chronic total coronary occlusions. | ||||||||
| 02 | specific | vessel diameters of ≥ 2.25 mm to ≤ 5.25 mm | ||||||||
| 03 | contraindication(s) | patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or the post-procedural antiplatelet regimen.
Patients with hypersensitivity or contraindication to everolimus or structurally-related compounds, or known hypersensitivity to stent components (cobalt, chromium, nickel, tungsten, methacrylic polymer, fluoropolymer), or with contrast hypersensitivity. |
||||||||
| 04 | implant description | ◊◊◊◊◊ | ||||||||
| 05 | specific attributes | ◊◊◊◊◊ | ||||||||
| 06 | material(s) | metal (n/a), everolimus, fluoropolymer coating | ||||||||
| 07 | implant surface | n/a | ||||||||
| 08 | key literature | ◊◊◊◊◊ | ||||||||
| 09 | notes about the company | n/a | ||||||||
| 10 | approvals | n/a | CE-Marking | X | FDA-Approval | |||||
| n/a | ISO | n/a | DIN | |||||||
| n/a | ASTM | n/a | CDSCO | |||||||
| n/a | CFDA | n/a | Patent | |||||||
| n/a | clean implant | n/a | ||||||||
| ♦ | current as of | 2022-07-25 | ||||||||
| unregistered / this page is not approved by the provider / unregistered implants have no professionnal lines or advertising | ||||||||||
| This “Implant Profile” shows a non-registered implant. This is a most comprehensive description of the important parameters of an implant. Information given here are checked thoroughly by “Implant-Register” and is not approved by the manufacturer/the company. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Implants may not be available in all countries. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | ||||||||||
| No comments yet. You are welcome to send comments or experiences. HERE, if you received an implant. and HERE, if you placed an implant. |
||||||||||
| If you have received this implant, you can register it HERE. | ||||||||||
