SYNCHRONY 2 Cochlear Implant System
IMPLANT PROFILE |
unregistered register here update records |
|||||||
| 01 | application | for individuals with severe-to-profound sensorineural hearing loss | ||||||
| 02 | specific | a cochlear implant (CI) system consists of two parts: an externally worn audio processor, which sits comfortably behind or off the ear, and an internal cochlear implant, which is surgically placed just under the skin. | ||||||
| 03 | company | MED EL | ||||||
| 04 | illustration | 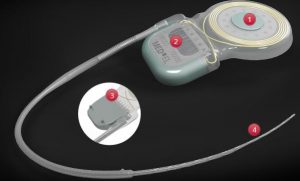 |
||||||
| implant | ||||||||
| 05 | material | n/a | ||||||
| 06 | approvals | X | CE-label (MDR 2021) | n/a | FDA-approval | |||
| learn more about approvals, certifications, authorities | ||||||||
| No comments yet. You are welcome to send comments or experiences. HERE, if you received this implant. and HERE, if you placed this implant. |
||||||||
| Received this implant? You can register it HERE (fee). Placed this implant? You can register it HERE for your patient (fee). |
||||||||
| current as of 2023-08-25 | ||||||||
| unregistered / this page is not approved by the provider / unregistered implants have no professionnal lines or advertising | ||||||||
| This “Implant Profile” shows a non-registered implant. This is a most comprehensive description of the important parameters of an implant. Information given here are checked thoroughly by “Implant-Register” and is not approved by the manufacturer/the company. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Implants may not be available in all countries. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | ||||||||
