Essure®
PROFILE |
 discontinued discontinued |
unregistered register here |
|||||||
| 01 | application | permanent birth control | |||||||
| 02 | specific | An Essure® insert is permanently placed into each of the fallopian tubes by a doctor. These inserts form a natural barrier that keeps sperm from reaching the eggs, preventing pregnancy. | |||||||
| 03 | company | Bayer AG | |||||||
| 04 | illustration | 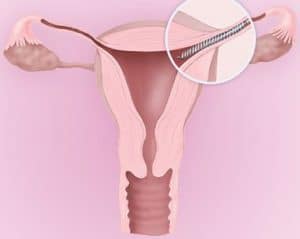 |
|||||||
| 05 | material | polyester fibers, nickel, titanium, platinum, silver-tin, stainless steel, and other | |||||||
| 06 | approvals | X | CE-Marking | X | FDA-Approval 2002 | ||||
| n/a | ISO | n/a | DIN | ||||||
| 07 | Bayer has made a business decision to voluntarily discontinue sales and distribution of Essure in the U.S. after December 31, 2018. “We made this decision based on a decline in Essure sales, and the conclusion that the Essure business is no longer sustainable. There has been no change in the positive safety and efficacy profile of Essure, and the U.S. Food & Drug Administration has maintained for several years that the benefits of Essure outweigh its risks.” | ||||||||
| 07 | regional directions | n/a | |||||||
| 08 | last update | 2019-03-14 | |||||||
| not registered / this page is not approved by the provider | |||||||||
| “Brief Profile” shows a nonregistered implant. Information given here are checked thoroughly by “Implant-Register”. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | |||||||||
| No comments yet. | |||||||||
