creos™ xenogain collagen
BRIEF PROFILE |
reg.: n/a register now ⇒ profile ⇒ full |
|||||||
| 01 | application | GTR, GBR™ for socket preservation | ||||||
| 02 | specific | combination of creos™ xenogain and porcine collagen type I | ||||||
| 03 | company | Nobel Biocare Services AG | ||||||
| 04 | illustration | 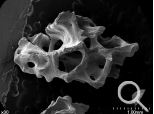 |
 |
|||||
|
implant |
|
surface |
||||||
| 05 | material | 90% bovine, 10% porcine | ||||||
| 06 | approvals | X | CE-Marking | n/a | FDA-Approval | |||
| n/a | ISO | n/a | DIN | |||||
| 07 | regional directions | n/a | ||||||
| 08 | last update | 2019-02-05 | ||||||
| not registered / this page is not approved by the provider | ||||||||
| “Brief Profile” shows a nonregistered implant. Information given here are checked thoroughly by “Implant-Register”. Illustrations refer to the company, if not shown differently. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. If there are pictures like logos or implants, they are taken from the companies website. The publishing company may inform us to hide them. | ||||||||
| No comments yet. | ||||||||
