BEGO OSS (discontinued)
|
unregistered |
||||||||
Brief Profile |
||||||||
| 01 | application | oral hard tissue | ||||||
| 02 | specific | osteoconductive, interconnecting pores, hydrophilic | ||||||
| 03 | company | BEGO Implant Systems GmbH & Co. KG | ||||||
| 04 | illustration | 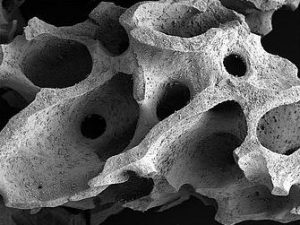 |
||||||
| 05 | material | bovine bone substitute | ||||||
| 06 | year of introduction | n/a | ||||||
| 07 | approvals | X | CE-Label | n/a | FDA-approval | |||
| n/a | double-blind test | n/a | prospective randomized controlled clinical study | |||||
| n/a | Other | |||||||
| 08 | last update | 2017-02-21 | ||||||
|
this page is not approved by the provider |
||||||||
| Information given here are checked thoroughly by “Implant-Register”. However we can not guarantee for any material or properties. Ask the manufacturer for such questions and give us the information to improve the Register. | ||||||||
